BY  GENN
GENN
2024/05
Blog
How Do You Get Magnesium From Electrolysis?
Introduction to the Electrolysis Process for Extracting Magnesium
Electrolysis is a chemical process that harnesses electrical energy to drive non-spontaneous reactions by using an external electric current. In the case of magnesium extraction through electrolysis, either magnesium chloride (MgCl2) or magnesium oxide (MgO) serves as the feedstock material. These feedstocks undergo specific chemical treatments prior to electrolysis to ensure optimal conversion rates during the process.
Magnesium Extraction via Electrowinning
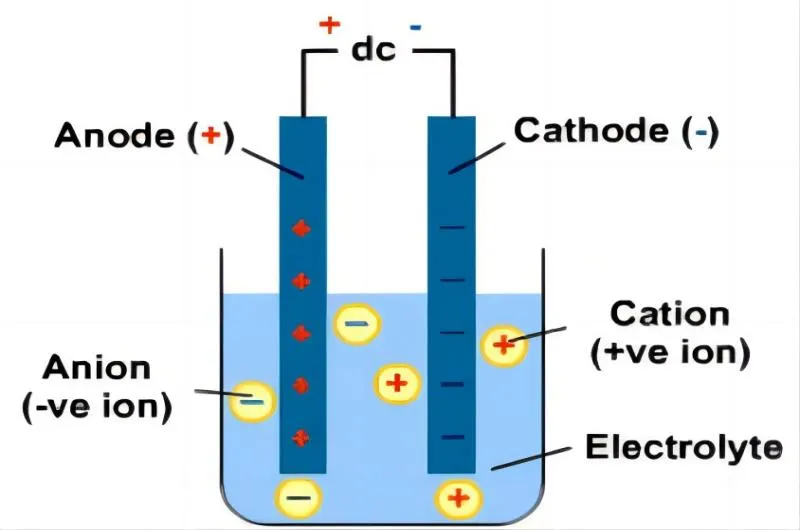
The electrolysis process utilized for extracting magnesium is known as electrowinning. It involves setting up an electrolytic cell comprising two electrodes immersed within a molten salt bath acting as the electrolyte medium.
The cathode (negative electrode) consists of molten magnesium ions, while graphite typically serves as the anode (positive electrode). When an electric current passes through this system from an external power source (DC supply), reduction takes place at the cathode, where positively charged Mg2+ ions gain electrons from this electrode surface, converting it into elemental magnesium (Mg).
Simultaneously at the anode, Cl- ions oxidize into chlorine gas (Cl2). This extraction method ensures high purity levels of obtained metallic magnesium, suitable for various industrial applications.
Importance of Magnesium Extraction for Various Industries
Vital Role in the Automotive Sector
Magnesium’s significance lies in its ability to contribute towards reducing vehicle weight without sacrificing structural integrity or safety standards. As automotive manufacturers strive towards improved fuel efficiency and reduced emissions, incorporating lightweight materials like magnesium becomes crucial. Employing advanced extraction techniques such as electrolysis to obtain high-quality metal with superior mechanical properties facilitates efficient production processes within this sector.
Aerospace Advancements with Magnesium
In aerospace engineering, where weight reduction plays a critical role in flight performance optimization while ensuring passenger safety remains paramount; utilizing materials like magnesium presents significant advantages. Electrolytic extraction methods play a vital role here by providing aerospace manufacturers with access to superior quality metal suitable for building lighter yet robust aircraft components that meet stringent industry standards.
The Process of Magnesium Extraction through Electrolysis
Preparation of magnesium chloride or oxide as the feedstock
Before starting the electrolysis process, it is crucial to prepare the feedstock, which is usually magnesium chloride (MgCl2) or magnesium oxide (MgO). These compounds serve as the source of magnesium ions during electrolysis. Magnesium chloride can be obtained from seawater or brines, while magnesium oxide is derived from magnesite ore.
The raw materials undergo a series of purification steps to remove impurities such as calcium and other metals. Once purified, the magnesium chloride or oxide is ready to be used in the electrolytic cell.
Setting up the electrolytic cell for magnesium extraction
The electrolytic cell used for extracting magnesium consists of several key components. The first crucial aspect is selecting suitable electrodes, with graphite being a common choice due to its resistance to chemical reactions and high melting point. The anode (positive electrode) and cathode (negative electrode) are immersed in an electrolyte solution within the cell.
Initiation of the electrolysis process by applying a current
Once all components are prepared and set up within the cell, including suitable electrodes immersed in molten salt electrolytes, an electric current is applied to initiate the extraction process. Through this application of direct current (DC), two main reactions occur simultaneously at different electrodes:
- . Reduction reaction at the cathode: At the cathode (-), positively charged Mg2+ ions migrating towards it are reduced by gaining two electrons each. This reduction reaction transforms Mg2+ into pure metallic magnesium (Mg).
2 . Oxidation reaction at the anode: At the anode (+), negatively charged Cl- ions migrate towards it and undergo oxidation by giving out one electron each. This oxidation reaction results in chlorine gas formation (Cl2).
As these reactions continue over time with continuous current flow, pure metallic magnesium accumulates at the cathode while chlorine gas evolves at the anode. By understanding these steps involved in extracting magnesium through electrolysis – from preparing feedstock and setting up appropriate electrodes and molten salt solutions to initiating electrochemical reactions – we gain insights into this fascinating industrial process that contributes significantly to various industries’ demands for this valuable metal.
Challenges and Innovations in Magnesium Electrolysis
Exploring Common Challenges in Magnesium Extraction via Electrolysis
Magnesium extraction through electrolysis, despite its effectiveness, is not without its challenges. One of the primary obstacles encountered is the high energy consumption associated with the process. The production of magnesium requires a significant amount of electrical energy, making it energetically intensive. This poses economic and environmental concerns as it contributes to increased costs and a higher carbon footprint.
Another challenge lies in the purity of the obtained magnesium. During electrolysis, impurities present in the feedstock can contaminate the resulting product. These impurities may affect the quality and mechanical properties of magnesium, making it less desirable for certain applications. Therefore, achieving high-purity magnesium through electrolysis remains an ongoing challenge that researchers and industry experts are actively addressing.
Furthermore, electrode degradation is a recurring issue in magnesium electrolysis. The intense conditions within the electrolytic cell can cause structural damage to electrodes over time due to corrosion or physical erosion. This deterioration hampers efficiency and increases maintenance costs. Developing durable electrode materials that can withstand harsh conditions while maintaining a high level of electrochemical activity is an area where innovative solutions are being explored.
Driving Innovations in Magnesium Electrolysis
To overcome these challenges, researchers are continuously exploring innovative techniques and technologies for magnesium extraction via electrolysis.
One approach involves optimizing cell design and operating parameters to enhance energy efficiency and reduce overall power consumption during production processes. Advancements are also being made to improve purity levels by employing various purification methods after initial extraction through electrolysis.
Techniques such as vacuum distillation or fractional crystallization can help remove impurities from molten magnesium to achieve desired purity levels for specific applications. Innovations also extend to electrode materials for increased durability.
Researchers are investigating alternative electrode compositions or protective coatings that can resist corrosion or erosion caused by harsh operating conditions within the electrolytic cell. These developments aim to extend electrode lifespan, improve process efficiency, and reduce maintenance requirements.