BY  GENN
GENN
2024/05
Blog
What Happens To Magnesium In Electrolysis?
Overview of Electrolysis
Explanation of the Process
Electrolysis is a chemical process that uses an electric current to drive a non-spontaneous chemical reaction. In this process, an electrically conductive substance, known as an electrolyte, is dissolved in a solvent or melted to create a conducting medium.
When an electric potential is applied across the electrodes immersed in the electrolyte solution, oxidation occurs at the anode (positive electrode) and reduction occurs at the cathode (negative electrode). This results in the decomposition of the electrolyte into its constituent elements or ions, or the production of a desired product.
Role of Electrodes and Electrolyte Solution
The electrodes play a crucial role in the electrolysis process. They provide the surface for the oxidation and reduction reactions to occur. The anode attracts negatively charged ions from the electrolyte solution, which then gain electrons and are oxidized.
Conversely, the cathode attracts positively charged ions from the electrolyte solution, which then lose electrons and are reduced. The electrolyte solution serves as the medium that allows the flow of ions between the electrodes, facilitating the transfer of electrons and the overall electrolysis reaction.
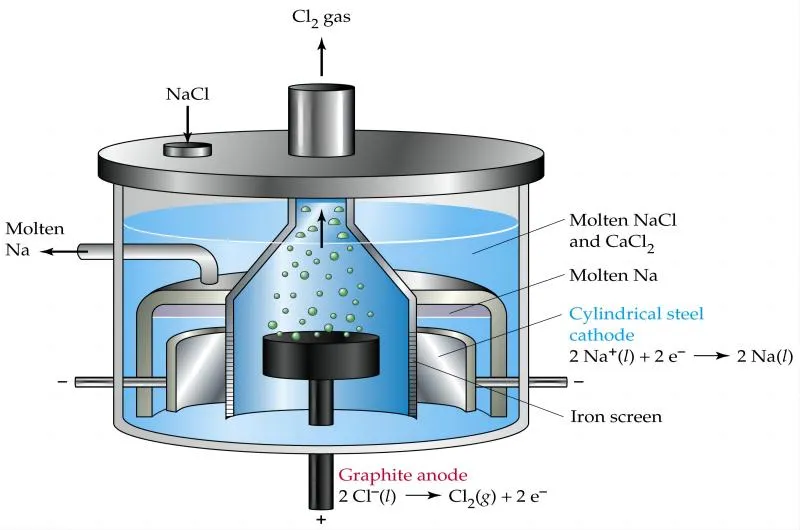
In the context of magnesium electrolysis, the electrolyte solution typically contains magnesium ions (Mg²⁺) and other ions that may be present in the magnesium compounds being processed. The choice of electrolyte solution depends on the specific application and the desired outcome of the electrolysis process.
The electrodes used in magnesium electrolysis are often made of a conductive material, such as graphite or metal, that can withstand the high temperatures and reactivity of magnesium. It is important to select electrodes that are compatible with the electrolyte solution and can withstand the chemical and thermal conditions of the electrolysis process.
Factors Influencing Magnesium’s Behavior in Electrolysis
The Impact of Current Intensity and Duration
Current intensity refers to the amount of electrical current passing through the electrolytic cell per unit of time, while duration indicates the length of time for which the electrolysis is carried out. Higher current intensities can lead to faster reactions at the electrodes, influencing the rate at which magnesium ions are oxidized at the anode and hydrogen gas is produced at the cathode. Longer durations of electrolysis can result in more extensive electrode reactions, potentially affecting the overall efficiency of magnesium extraction.
Moreover, variations in current intensity and duration can impact not only the rate of reaction but also the distribution of products formed during electrolysis. For instance, if an excessively high current is applied for prolonged periods, it may cause overheating within the cell, leading to undesirable side reactions or decomposition of electrolyte solutions.
By precisely regulating current intensity and duration in response to changing conditions within the electrolytic cell, it becomes possible to achieve greater control over magnesium’s behavior during electrolysis. This adaptive approach holds promise for improving overall process efficiency and ensuring consistent production outcomes with minimal environmental impact.
The Role of Concentration and Temperature
The concentration and temperature of the electrolyte solution are key factors influencing how magnesium behaves during electrolysis. The concentration refers to the amount of dissolved ions present in the solution—a parameter that directly affects conductivity and reaction rates within the cell. Higher concentrations typically result in increased ion mobility, facilitating faster transfer of magnesium ions towards respective electrodes for oxidation or reduction processes.
This aspect becomes particularly relevant when considering production scalability and optimizing resource utilization in industrial-scale electrolytic operations. Additionally, temperature exerts a significant influence on the reaction kinetics and thermodynamic considerations governing magnesium’s behavior in electrochemical environments.
Elevated temperatures can accelerate reaction rates by providing additional thermal energy needed to overcome activation barriers associated with electrode processes. However, extreme temperatures may also pose challenges such as increased energy requirements for maintaining optimal operating conditions or potential degradation of electrode materials due to thermal stress effects.
Furthermore, balancing concentration levels with temperature control represents a delicate optimization challenge that requires careful consideration when designing electrolytic systems for magnesium extraction applications. Strategies aimed at achieving a harmonious interplay between these two factors involve thermal management techniques like efficient heat dissipation mechanisms or controlled cooling strategies that help maintain stable operating conditions throughout extended periods of electrochemical processing.
Applications and Uses of Magnesium in Electrolysis
Production of Pure Magnesium Metal
Magnesium is a crucial metal with a wide range of industrial applications due to its lightweight, high strength-to-weight ratio, and excellent corrosion resistance. Electrolysis plays a vital role in the production of pure magnesium metal.
The process involves extracting magnesium from its compounds, such as magnesium chloride or magnesium sulfate solutions, through the application of an electric current. This electrolytic extraction method allows for the production of high-purity magnesium, which is essential for various industries, including aerospace, automotive, and electronics.
Electrolytic Extraction from Magnesium Chloride or Magnesium Sulphate Solutions
In the electrolytic extraction of magnesium, magnesium chloride or magnesium sulfate solutions serve as the electrolyte. These solutions are subjected to electrolysis, where an electric current is passed through them to facilitate the decomposition of the magnesium compound.
During this process, magnesium ions migrate towards the cathode, where they are reduced to form pure magnesium metal. The electrolytic extraction from magnesium chloride or magnesium sulfate solutions ensures a controlled and efficient method of obtaining magnesium with high purity levels, suitable for demanding industrial applications.
Purification Process to Remove Impurities
One of the critical steps in the production of pure magnesium metal through electrolysis is the purification process to remove impurities. Impurities present in the magnesium compound can affect the quality and properties of the final product.
Through various purification techniques, such as fractional crystallization or solvent extraction, impurities like calcium, aluminum, and iron are separated from the magnesium metal. This purification process ensures that the magnesium obtained through electrolysis meets the stringent quality standards required for its diverse applications, guaranteeing optimal performance and reliability in end-use products.
Challenges and Limitations in Magnesium Electrolysis
High Energy Consumption Due to High Melting Point
Magnesium has a relatively high melting point of 650 degrees Celsius, which poses a significant challenge in its electrolysis process. The high temperature required to melt magnesium increases the energy consumption during electrolysis. The resistance to heat flow leads to an increase in electrical resistance, necessitating higher electrical currents and longer electrolysis durations to achieve the desired level of extraction.
This results in increased power consumption, making magnesium electrolysis an energy-intensive process. To address this challenge, researchers are exploring innovative techniques such as using alternative electrode materials or modifying the electrolyte composition to reduce the energy requirements.
Corrosion Issues with Electrodes Due to Highly Reactive Nature
Magnesium is highly reactive, making it prone to corrosion when exposed to certain electrode materials and electrolyte solutions used in the electrolysis process. The corrosive nature of magnesium arises from its tendency to form strong chemical bonds with other elements. During the anode reaction, where magnesium ions are oxidized, this reactivity can lead to the erosion of electrodes and result in decreased efficiency and lifespan of the equipment.
Researchers are working on developing corrosion-resistant electrode materials that can withstand prolonged exposure to reactive magnesium ions. Coating electrodes with protective layers or using alloys with improved resistance against corrosion are some solutions being explored.
The Need for Efficient Recycling Methods
Another significant challenge in magnesium electrolysis lies in finding efficient recycling methods for spent electrodes and unused electrolytes. As electrodes corrode over time due to repeated use, they become less effective and require replacement. Additionally, spent or contaminated electrolytes further add to environmental concerns if not properly managed or recycled.
Developing effective recycling processes is crucial for reducing waste generation and minimizing the environmental impact associated with magnesium production through electrolysis. Research efforts are focused on finding sustainable methods that allow for efficient recovery of valuable resources from spent electrodes and purification of used electrolytes for reuse.
High energy consumption due to a high melting point and corrosion issues posed by its highly reactive nature present significant challenges in magnesium electrolysis processes. Addressing these challenges requires innovative approaches such as alternative electrode materials, modifications in the composition of the electrolyte solution, or coating techniques that enhance corrosion resistance while reducing energy requirements.
Additionally, developing efficient recycling methods is essential for sustainability by minimizing waste generation associated with spent electrodes and contaminated/unused electrolytes. Overcoming these limitations will contribute to optimizing the production processes involved in extracting pure magnesium metal through electrochemical means.