BY  GENN
GENN
2024/07
Blog
What Is Unusual About Silicon?
Unusual Characteristics of Silicon
Elemental Properties: A Comparative Study with Carbon
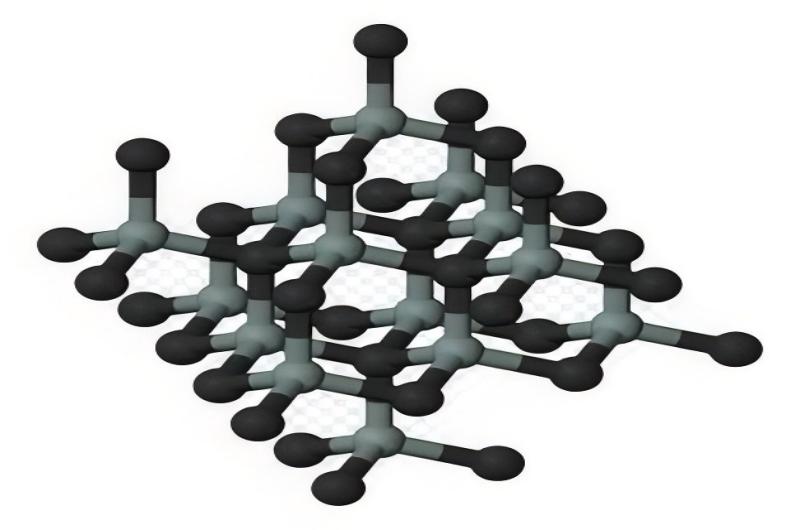
Silicon shares a neighborhood in the periodic table with carbon, often termed its “elemental cousin.” While carbon is famous for its versatility in forming complex organic molecules, silicon exhibits a different kind of versatility due to its ability to form robust crystalline structures and act as a semiconductor. Silicon’s abundance in the Earth’s crust, about 27.7% by mass, underscores its pervasive presence in terrestrial geology and its crucial role in shaping planetary landscapes.
Semiconductor Properties: The Silicon Revolution
Silicon’s semiconductor properties are pivotal to the modern electronics revolution. As a semiconductor, silicon can be modified by introducing minute impurities, a process known as doping, to control its electrical conductivity.
This capability forms the basis of transistors, the building blocks of digital circuits, enabling the rapid miniaturization and increased performance of electronic devices over the decades. The reliability and scalability of silicon technology have propelled advancements in computing power, telecommunications, and beyond, shaping the very fabric of our interconnected world.
Silicon‘s Role in the Semiconductor Industry
Silicon is the foundation of the semiconductor industry, primarily due to its one-of-a-kind electrical properties and plentiful availability. Its ability to perform electricity under particular problems while working as an insulator under others makes it an excellent product for digital elements. This double feature, referred to as semiconducting, permits silicon to be utilized in the manufacturing of transistors, which are the basic structure blocks of all modern electronic tools.
The process of transforming silicon right into semiconductors includes numerous crucial actions, including purification, crystal growth, and doping. High-purity silicon is crucial, and it is generally obtained with the Czochralski procedure, which produces big, single-crystal silicon ingots. These ingots are after that sliced right into thin wafers and based on a series of chemical treatments and doping procedures, where contaminations are purposefully presented to regulate the electric residential properties of the silicon. This accurate control over electric conductivity is what enables the creation of complex electronic circuits.
Silicon’s dominance in the semiconductor sector is also associated with its outstanding thermal stability and oxide layer development. Silicon dioxide (SiO ₂), which normally based on silicon surface areas, functions as an efficient insulator and protective layer, making silicon-based tools extremely dependable and sturdy. Moreover, silicon’s compatibility with existing manufacturing technologies and facilities seals its function as the material of choice for semiconductors.
The prevalent usage of silicon in semiconductor innovation has driven improvements in various areas, including computer, telecommunications, and consumer electronics. From microprocessors and memory chips to sensing units and solar batteries, silicon’s versatile applications remain to drive development and technical development. As the demand for quicker, smaller, and more efficient digital devices grows, recurring r & d in silicon modern technology continues to be vital to meeting these obstacles.
Wealth and Natural Occurrence of Silicon
Silicon is the 2nd most bountiful component in the Earth’s crust, gone beyond just oxygen. It comprises roughly 27.7% of the crust by weight. This prevalence results from silicon’s amazing capability to develop substances with various other elements, particularly oxygen, causing numerous silicates and oxides.
Silicon is hardly ever located in its pure type in nature. Instead, it predominantly exists as a component of minerals. Typical minerals including silicon consist of quartz (SiO ₂), feldspar, and mica. These minerals are basic parts of rocks such as granite and sandstone.
The removal and improvement of silicon are critical for its industrial applications. The key method of acquiring pure silicon entails the reduction of silicon dioxide (sand) with carbon in an electric arc furnace. This procedure yields metallurgical-grade silicon, which is then additionally refined to create semiconductor-grade silicon.
Despite its wealth, the reliable manufacturing of high-purity silicon calls for innovative modern technology and substantial power input. This requirement underpins the economic and critical value of silicon in modern technology, particularly in the semiconductor industry, where its special buildings are harnessed for electronic tools.
The Environmental Impact of Silicon Production
The manufacturing of silicon, while important to modern-day technology, features significant environmental concerns. Silicon itself is just one of one of the most plentiful components on Earth, mostly extracted from quartz and other silica-containing minerals. However, the process of refining and detoxifying silicon for industrial applications, particularly for the semiconductor market, includes significant energy consumption and making use of possibly dangerous chemicals.
The preliminary action in creating high-purity silicon is the decrease of quartz in electrical arc heaters, which requires a considerable quantity of electricity, usually acquired from non-renewable sources. This energy-intensive process adds to greenhouse gas exhausts, posing an obstacle to initiatives focused on decreasing carbon impacts.
Along with power usage, the use of chemicals such as hydrochloric acid and trichlorosilane in the purification stages raises environmental problems. These materials, if not taken care of correctly, can lead to dirt and water contamination. The waste products from silicon wafer manufacturing likewise existing disposal obstacles, necessitating rigorous waste administration methods to minimize their ecological effect.
Additionally, the mining of silica sand, a key basic material for silicon production, can result in habitat destruction and biodiversity loss. Mining activities commonly result in landscape changes and the disturbance of regional communities. Sustainable mining methods and the growth of alternative materials are consequently necessary to minimize the ecological footprint of silicon manufacturing.
Efforts to minimize these impacts consist of the adoption of renewable resource resources in the production procedure, improved recycling strategies for silicon waste, and the advancement of much less unsafe chemical choices. Advances in innovation and development continue to drive the market towards even more lasting techniques, aiming to balance the growing need for silicon with environmental stewardship.
Advancements and Future Potential of Silicon
Silicon’s special chemical residential or commercial properties and its crucial role in the semiconductor market have actually driven various technologies and opened vast future potential. One essential location of advancement lies in the development of silicon-based photonics, where silicon is used to control and transmit light, leading the way for faster and much more effective information transmission. This innovation is critical for the future of high-speed web and progressed computer systems.
Another amazing advancement is the integration of silicon in quantum computers. Silicon’s capacity to sustain qubits– standard devices of quantum details– offers a path towards even more secure and scalable quantum computers. Researchers are actively exploring silicon-based quantum dots and transistors to develop sensible and reputable quantum tools.
In the realm of power, silicon remains to be crucial. Silicon anodes are being boosted to boost the performance of lithium-ion batteries, raising energy density and prolonging battery life. These improvements are crucial for the future generation of electrical lorries and portable electronic devices.